
News

Leap Therapeutics' Board of Directors Member Dr. Monica Bertagnolli Appointed as the Director of the National Cancer Institute
Leap Therapeutics, Inc., a biotechnology company focused on developing targeted and immuno-oncology therapeutics today announced that its Board member, Monica Bertagnolli, MD, has been appointed as the 16th Director of the National Cancer Institute (NCI) by the Biden administration.

Context Therapeutics and The Menarini Group Announce Clinical Trial Collaboration and Supply Agreement to Evaluate ONA-XR and Elacestrant Combination
Context Therapeutics, a women’s oncology company developing small molecule and immunotherapy treatments for breast and gynecological cancers, and The Menarini Group today announced a clinical trial collaboration and supply agreement for Menarini’s oral selective estrogen receptor degrader (SERD), elacestrant.

VAXXAS Patch Worked For All COVID Strains
A protein-based vaccine patch has proved effective against all major variants of Covid when given to mice, say researchers. Vaxxas is an Australian biotechnology company which is developing patch dispensers for different diseases.

Leap Therapeutics Announces Initiation of New DKN-01 Clinical Trials in Gastric Cancer, Colorectal Cancer and Endometrial Cancer
Leap Therapeutics and BeiGene today announced the initiation of Part C of the ongoing DisTinGuish study to evaluate DKN-01, Leap's anti-SSEDickkopf 1 (DKK1) antibody, in combination with tislelizumab, BeiGene's anti-PD-1 antibody, and chemotherapy compared to a tislelizumab and chemotherapy control arm, in patients with gastric or gastroesophageal junction cancer (G/GEJ).

Vaxxas and University of Sydney complete study assessing needle-free HD-MAP technology for vaccine administration
Australian biotechnology company, Vaxxas Pty Ltd (Vaxxas), in collaboration with the University of Sydney Institute for Infectious Diseases, Faculty of Medicine and Health, has completed a study assessing the usability and acceptability of its novel High-Density Microarray Patch (HD-MAP) to administer vaccines.

Context Therapeutics® Announces Encouraging Preclinical Data from Two Programs to be Presented at the American Association for Cancer Research (AACR) Annual Meeting 2022
Lead clinical-stage oral progesterone receptor (PR) antagonist ONA-XR demonstrates strong efficacy across multiple PR+ solid tumor models.

Vaxxas Licenses HexaPro Spike Glycoprotein from University of Texas at Austin for Use on its Needle-Free Vaccine Patches
Vaxxas’ proprietary HD-MAP patch has been designed to eliminate the need for refrigerated distribution and to allow for administration by individuals with minimal training, or even self-administration, making distribution during a pandemic more efficient.

Leica Biosystems and Leap Therapeutics Partner on Companion Diagnostic to Advance Care for Cancer Patients
Leica Biosystems, a cancer diagnostics company, has entered into an agreement with Leap Therapeutics (NASDAQ: LPTX), a biotechnology company, to develop a companion diagnostic to detect Dickkopf-related protein 1 (DKK1).

Context Therapeutics® Announces Positive Data from ONA-XR in Early Breast Cancer at 2021 San Antonio Breast Cancer Symposium
Context Therapeutics Inc. (Nasdaq: CNTX), a women’s oncology company developing small molecule and immunotherapy treatments for breast and gynecological cancers, today announced that data from the window-of-opportunity clinical trial of onapristone extended release (ONA-XR).

I2020 ACCELERATOR ANNOUNCES THE LAUNCH OF GWYNANT THERAPEUTICS AND CELYN THERAPEUTICS TO DEVELOP NOVEL SMALL MOLECULES FOR ONCOLOGY TARGETS
i2020, an accelerator program backed by the San Diego-based venture company Torrey Pines Investment, has announced two new start-up investments together with OrbiMed Advisors.

Preclinical Studies From Vaxxas and Collaborators Reveal Potential of Vaxxas’ Novel High-Density Microarray Patch (HD-MAP) to Effectively Deliver SARS-CoV-2 Spike Vaccine
Complete protection from COVID-19 disease by a single dose skin patch delivery using Vaxxas’ HD-MAP was demonstrated in a lethal challenge of the SARS-COV-2 virus in an animal model.

Preclinical Studies From Vaxxas and Collaborators Reveal Potential of Vaxxas’ Novel High-Density Microarray Patch (HD-MAP) to Effectively Deliver SARS-CoV-2 Spike Vaccine
Vaxxas, a clinical-stage biotechnology company commercializing a novel vaccination platform, today announced that research demonstrating the potential of Vaxxas’ novel high-density microarray patch (HD-MAP) for vaccination against COVID-19 had been published in a manuscript posted to the preprint server BioRxiv.

Avelas Biosciences Presents Phase 2, Period 2 Data in Virtual Poster Session at the 22nd Annual Meeting of The American Society of Breast Surgeons
Avelas Biosciences, Inc., a pioneer in the field of fluorescence imaging for real-time cancer detection, presented the results from Period 2 of its most recent clinical trial in a virtual poster session at the 22nd Annual Meeting of The American Society of Breast Surgeons.
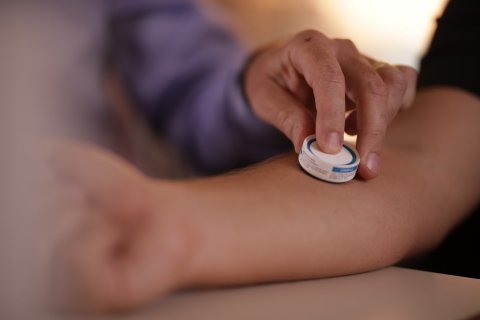
Vaxxas Announces $22 Million Award from U.S. Government to Advance Vaxxas Needle-Free HD-MAP™ Vaccine Patch Technology for Pandemic Response
Needle-free Vaxxas HD-MAP patch offers potential platform to dramatically improve vaccination in pandemic response by producing more patient-doses from limited quantities of vaccine, enabling rapid vaccine distribution without refrigeration, and creating a novel option for self-administration.
